Tackling cancer by leveraging multiple modalities
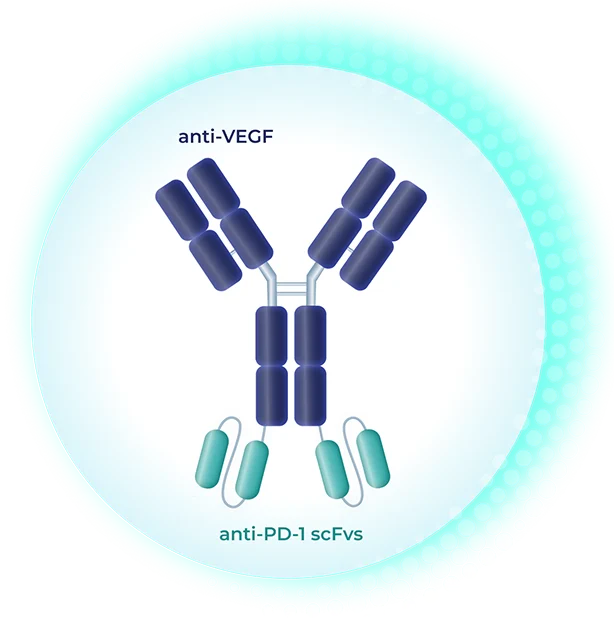
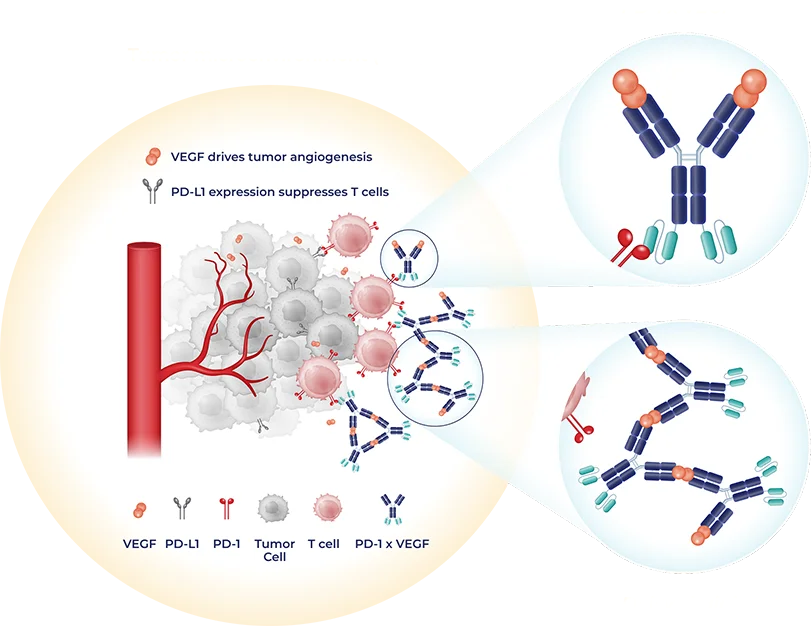
Our oncology programs encompass multiple modalities, including a PD-1 x VEGF bispecific antibody that we see as a foundational backbone, as well as antibody-drug conjugates (ADCs) that could be utilized either alone or in combination with our immunotherapy candidate. We’re working smartly and efficiently by leveraging established targets and validated biology, with the goal of advancing rapidly towards multiple solid tumor indications.
Programs with potential for monotherapy or combination therapy across multiple solid tumor indications
INDICATIONS
REGION
other solid
tumors
 Global
Global (Ex-China)
 Greater China
Greater Chinatumors
 Global
Global (Ex-China)
 Greater China
Greater Chinatumors
 Global
GlobalTopoli: Topoisomerase 1 inhibitor; Ex-China refers to Ex-Greater China.